As part of the European project “Harmonization of the Albanian Pharmaceutical Legislation with the European Pharmaceutical Directive” (PHARM-EU), included in the Jean Monnet Module actions and funded by the EU’s Erasmus+ programme, the conference “Clinical Trials and Access to Medicines: Challenges, Regulations and Innovation” was held on April 10, 2025, with the participation of professionals from the field.
The scientific organizer of the conference, Assoc. Prof. Malvina Hoxha, opened the event by highlighting the crucial value of clinical trials for public health and the promotion of therapeutic innovation, while also pointing out the regulatory challenges in non-EU countries such as Albania.
The Dean of the Faculty of Pharmacy, Prof. Roberto Perrone, emphasized the fundamental role of pharmacists in conducting clinical trials, not only as professionals involved in drug management, but also as key figures in patient communication and clinical data collection.
Among the speakers, Prof. Nunzio Denora presented the Pilot Clinical Study of a new pediatric formulation for the treatment of eosinophilic esophagitis, a project that integrates pharmaceutical innovation, clinical research, and highlights the strong attention to the needs of pediatric patients.
Dr. Ermal Pashaj discussed the strategic role of universities in the implementation and operational support of clinical trials, presenting the experience of the “TumoCure Gel” clinical trial, applied to patients with progressive, late-stage head and neck squamous cell carcinoma.
Prof. Arben Boshnjaku outlined the current clinical trials regulatory framework in Kosovo, highlighting the central role of ethics committees in protecting trial participants and safeguarding the integrity of clinical research.
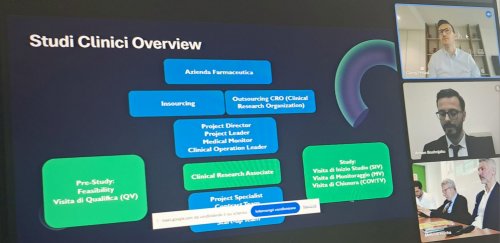
Dr. Gjergj Priska illustrated the role of the Clinical Research Associate, a professional responsible for monitoring clinical studies, underlining the importance of continuous training and effective communication between sponsors, clinical sites, and regulatory authorities.
At the conclusion of the conference proceedings, the scientific coordinator of the project, Prof. Assoc. Malvina Hoxha, emphasized the importance of strengthening the national regulatory framework, which is crucial to ensuring high standards of quality in clinical research, thereby ensuring the safety and effectiveness of treatments and the continuous progress of pharmacological therapies.